Picking a right catalyst for synthesis is vital to achieve optimum performance. Due to regulatory concerns and potential environmental threat of tin-based catalyst, many researchers are looking for better alternatives.
KEY INSIGHTS
• Recent trends in the Polyurethane Market
• Role of catalysts
• Key manufacturers of tin free catalysts
BULLET POINTS
• The reaction between polyols with polyisocyanates results in the formation of polyurethane.
• The Polyurethane Market size is estimated at USD 87.68 billion in 2025, and is expected to reach USD 113.84 billion by 2030, at a CAGR of 5.36% during the forecast period (2025-2030).
• The PU catalyst market is expected to grow at a CAGR of 6% during the period of 2023-2028.
• The use of organotins is restricted or regulated under various legislation in various countries.
• The manuscript lists the alternatives to tin-free catalysts.
KEYWORDS
Polyurethane, Catalysts, Ecological concerns, Alternatives to organotin catalysts
DETAILED REPORT
The reaction between polyols with polyisocyanates results in the formation of polyurethane [1]. The required set of properties of the product can be brought about by the judicious choice of reactants. This makes polyurethane an important polymer finding its application in wide array of fields (Figure 1). In case of foams the choice and ratio of blowing and gelling agents can significantly alter the properties of the product.
The Polyurethane Market size is estimated at USD 87.68 billion in 2025, and is expected to reach USD 113.84 billion by 2030, at a CAGR of 5.36% during the forecast period (2025-2030) [2]. If focused on the sector-wise division of market share then the Polyurethane Foam Market in India has reached US$ 2.8 billion in 2022 and is expected to reach US$ 5.18 billion by 2030 and is expected to grow with a CAGR of 8.0% during the forecast period 2023-2030 [3]. Another market research conducted by IMARC Group concluded that Indian polyurethane foam market size reached US$ 3.74 Billion in 2023. And it is expected that the market will reach US$ 6.76 Billion by 2032, exhibiting a growth rate (CAGR) of 6.30% during 2024 2032 [4]. India polyurethane synthetic leather market size was valued at USD 7.06 billion in 2023 and is projected to grow at a CAGR of 7.9% from 2024 to 2030 [5].
Besides the reactants, another important ingredient in the synthesis is catalyst. It is obvious that with the rise in PU market, the requirement of catalyst will also grow. The PU catalyst market is expected to grow at a CAGR of 6% during the period of 2023-2028 [6].
In general, the catalysts are classified as gelling catalyst and blowing catalyst. By definition gelling catalysts facilitates the reaction between isocyanates and alcohol producing urethane linkages whereas, the blowing catalysts are known for carrying out the reaction between isocyanate-blowing agent (Water is mostly used. However, other blowing agents include methylene chloride, trichlorofluoromethane, etc.) [7-8]. Both the catalyst can be used in combination based on the required set of properties of final product (for example in case of foam fabrication).
The presented manuscript highlights only on gelling catalysts. Organotin compounds such as dibutyltin dilaurate (DBTDL) and dioctyltin dilaurate (DOTDL) are most commonly used gelling catalyst due to their compatibility with many polyols and isocyanates along with reliability, strong performance and provide good drying times and mechanical properties. Apart from these organotin compounds, tertiary amines, carboxylic acid salts and other organometallics (Table 1) can also be used to catalyze the reaction of isocyanates and polyols.
Figure 1: Applications of PU [9]
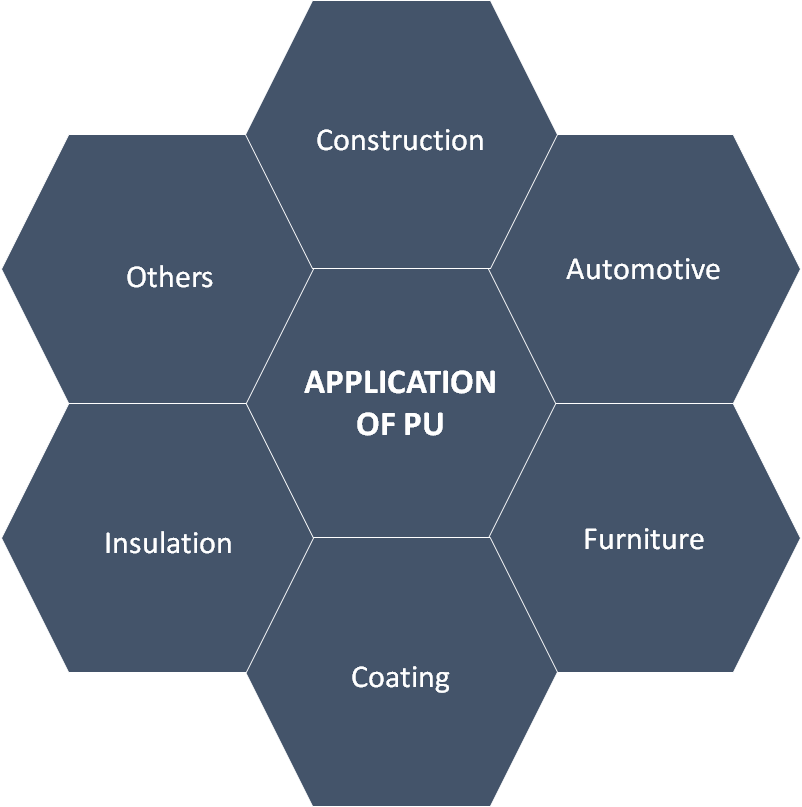
Table 1: List of organometallics other than tin-based compounds [9]
| Phenylmercuric propionate |
| Lead octoate |
| Potassium acetate/ octoate |
| Ferric acetylacetonate |
| Bismuth neodecanoate |
| Zinc neodecanoate |
ENVIRONMENTAL HAZARDS
Tin in its inorganic form is considered to be non-toxic, but the toxicological pattern of organotin compounds is complex. Depending on the nature and the number of organic groups bound to the Sn cation, some organotins show specific toxic effects to different organisms even at very low concentrations. Organotins are known to cause detrimental impact to aquatic ecosystems for example, it is reported that neogastropods in the regions of southwest of England were affected due to these chemicals that led to decline in its population. It leads to bioaccumulation in food chain. In mammals it deposits mostly in liver and kidneys, adversely affects skin, eyes, liver, CNS. It is also reported that it Inhibits mitochondria respiration by preventing the oxidation of keto acids. Tetra-organotin compounds produce muscular weakness, paralysis, respiratory failure, tremors, and hyperexcitability as acute effects in mice and dogs [10-12].
GOVERNMENT LEGISLATION ON THE USE OF ORGANOTIN COMPOUNDS
Under REACH Annex XVII restriction 20 in the EU, more than 0.1% weight of organotin is restricted in consumer products [13]. In Japan, the “Act on Control of Household Products Containing Harmful Substances (Act No. 112 of October 12, 1973)” restricts the use of tin compounds in household products [14-16]. “Korean Special Act on the Safety Control of Children’s Products” (Effective from 4th June 2015) restricts the use of Dibutyltin and Tributyltin compounds [17]. Australia has also regulated the exposure to organotin compounds under the “Workplace Exposure Standards for Airborne Contaminants” and “Australian Water Quality Guidelines for Fresh and Marine Waters” regulation [18].
LIST OF TIN FREE GELLING CATALYSTS
The following Table 2 shows the details of gelling catalysts along with their manufacturers/ suppliers and chemistry. The data presented in the following table are taken from the company’s website either from webpages or the available brochures.
Table 2: List of Gelling catalysts
| Company | Trade name | Chemistry |
| Umicore | Valikat Bi series | Bismuth neodecanoate |
| Valikat Zn series | Zinc neodecanoate | |
| Valikat BiZn series | Bismuth Zinc based | |
| Valirex Zn | Zinc neodecanoate | |
| Covestro AG | Desmorapid | |
| Kao corporation | Kaolizer | Tertiary amine |
| BASF | Lupragen® N 101 | N, N-Dimethylethanolamine |
| Lupragen® N 105 | N-Methylmorpholine | |
| Lupragen® N 201 | Triethylenediamine | |
| Lupragen® N 203 | Triethylenediamine | |
| Lupragen® N 400 | N,N,N’-Trimethylaminoethylethanolamine | |
| Lupragen® N 500 | N,N,N’,N’-Tetramethyl-1,6-hexanediamine | |
| Lupragen® N 700 | 1,8-Diazabicyclo-5,4,0-undecene-7 | |
| Tosoh corporation | TEDA® | Triethylenediamine |
| DMI | 1,2-Diemethylimidazole | |
| RZETA (Reactive catalyst) | ||
| RX4 (Reactive catalyst) | ||
| RX24 (Reactive catalyst) | Reactive amine blend | |
| RX210 (Reactive catalyst) | Reactive amine blend | |
| CX20 (Delayed action catalyst) | ||
| NCT (Delayed action catalyst) | ||
| TF (Delayed action catalyst) | ||
| B41 (Specialty catalyst) | ||
| CX60 (Specialty catalyst) | ||
| DB30 (Specialty catalyst) | ||
| DB60 (Specialty catalyst) | ||
| DM70 (Specialty catalyst) | 1,2-dimethylimidazole based; 30% ethylene glycol | |
| F10 (Specialty catalyst) | 1,2-dimethylimidazole based amine catalyst) | |
| F22 (Specialty catalyst) | ||
| F94 (Specialty catalyst) | ||
| M50 (Specialty catalyst) | ||
| MC12 (Specialty catalyst) | ||
| S10 (Specialty catalyst) | ||
| Huntsman International LLC | Z-80 (General Purpose Catalyst) | N, N, N-tris(3-dimethylaminopropyl) amine |
| ZR-40 (General Purpose Catalyst) | N,N,N’,N’’,N’’ – pentamethyl-dipropylene triamine | |
| DPA (Low emission catalyst/ Reactive catalyst) | N-(3-dimethylaminopropyl)-N, N-diisopropanolamine | |
| LE-220, LE-225, LE-310, LE-340, LE-355, LE-380, LE-529, LED-204, Z-131 (Low emission catalyst/ Reactive catalyst) | ||
| Z-130 (Low emission catalyst/ Reactive catalyst) | 1,3-propanediamine, N’-(3-(dimethylamino)propyl)-N,N-dimethyl | |
| Suzhou Xiangyuan New Materials Co. Ltd | MOCA-WH (MOCA-I) | 4,4’-methylene-bis(ortho-chloroaniline) |
| M-CDEA | 4,4’-methylene-bis(3-chloro-2,6-diethylaniline) | |
| ML-200 | 3-chloro-3’-ethyl-4,4’-diaminodiphenylmethane | |
| Evonik | DABCO® 33 LV | Catalyst based on triethylenediamine |
| DABCO® 33 LX | ||
| DABCO® 8174 | ||
| DABCO® CRYSTAL | ||
| DABCO® DC 5 LE | ||
| DABCO® MB 20 | Bismuth based | |
| DABCO® NE 1050 | ||
| DABCO® NE 1066 | ||
| DABCO® NE 1070 | ||
| DABCO® NE 1082 | ||
| DABCO® NE 1091 | ||
| DABCO® NE 1550 | ||
| DABCO® NE 1600 | ||
| DABCO® NE 2000 C | ||
| DABCO® XED 20 B | ||
| DABCO® EG | Gelling catalyst for MEG extended systems | |
| DABCO® 25 S | Gelling catalyst for BDO extended systems | |
| DABCO® NCM | ||
| POLYCAT® 203 | ||
| POLYCAT® DBU | ||
| POLYCAT® 9 | ||
| POLYCAT® 204 | ||
| KOSMOS® MB 16 | Bismuth carboxylate catalyst | |
| KOSMOS® MB 19 | Bismuth carboxylate catalyst | |
| KOSMOS® T 100 | ||
| Lanxess | Addocat® 117 | 1,4-Dimethylpiperazine |
| Addocat® DB | N,N- Dimethylbenzylamine | |
| Addocat® 105 | 32.8-33.8% solution of triethylenediamine in dipropylene glycol | |
| Momentive | EF-600 (Low emission catalyst) | Amine catalyst |
| EF-600S | ||
| EF-602 (Low emission catalyst) | Amine catalyst | |
| EF-620 (Low emission catalyst) | Amine catalyst | |
| EF-680 (Low emission catalyst) | Amine catalyst | |
| EF-700 | ||
| C-247 | Amine catalyst | |
| A-300 | Amine catalyst | |
| A-305 | Amine catalyst | |
| A-33 | ||
| A-33R | Amine catalyst | |
| C-28 | ||
| C-41 | ||
| MC-710 | Bismuth-based catalyst | |
| A-535 (Delayed action gel catalyst) | ||
| A-537 (Delayed action gel catalyst) | ||
| A-575 (DBU based temperature activated, delayed-action gelling-selective catalyst) | ||
| A-577 (Delayed action, gelling-selective catalyst) | ||
| LC-5636 | Copper based catalyst | |
| MC-810 | Tin-free metal-based catalyst | |
| Mofan Polyurethane Co. Ltd | Mofan 8 | N,N-dimethylcyclohexylamine |
| Mofan 41 | 1,3,5-tris[3-(dimethylamino)propyl]hexahydro-s-triazine | |
| Mofan TMR-30 | 2,4,6-tris(dimethylaminomethyl)phenol | |
| Mofan 77 | N-[3-dimethylamino)propyl]-N,N’,N’-trimethyl-1,3-propanediamine | |
| Mofan 33LV | 33% triethylenediamine in DPG | |
| Mofan DPA | N-(3-dimethylaminopropyl)-N,N-diisopropanolamine | |
| Mofan EG | For MEG extended systems | |
| Mofan S-25 | For BDO extended systems | |
| Borchers – A Milliken brand | Borchi® Kat 0243 | Based on Bismuth, Lithium |
| Borchi® Kat 0244 | Based on Bismuth, Zinc | |
| Borchi® Kat 0245 | Based on Zinc, Calcium | |
| Borchi® Kat 0761 | Based on Zinc neodecanoate | |
| Borchi® Kat 19 | Based on Zinc | |
| Borchi® Kat 21 | Based on Bismuth | |
| Borchi® Kat 22 | Based on Zinc | |
| Borchi® Kat 24 | Based on Bismuth octoate | |
| Borchi® Kat 315 | Based on Bismuth | |
| Borchi® Kat 315 EU | Based on Bismuth | |
| Borchi® Kat EP1 | Based on Zinc |
REFERENCES
[1] Polymer Materials, 7th edition, J. A. Brydson, Butterworth-Heinemann.
[2] https://www.mordorintelligence.com/industry-reports/polyurethane-market. Accessed on 01 February 2025.
[3] https://www.giiresearch.com/report/dmin1285091-india-polyurethane-foam-market.html Accessed on 01 February 2025.
[4] https://www.imarcgroup.com/india-polyurethane-foam-market. Accessed on 01 February 2025.
[5] https://www.grandviewresearch.com/industry-analysis/india-polyurethane-pu-synthetic-leather-artificial-leather-market. Accessed on 01 February 2025.
[6] A market report article on “Polyurethane catalyst market”. Link: https://www.marketsandmarkets.com/Market-Reports/polyurethane-catalyst-market-118466262.html?srsltid=AfmBOorEjXVJ9NMcSP-N8SGt_A1uwFtst8LAOV3XfTBGuuFxP-OvysMF. Accessed on 01 February 2025.
[7] Dworakowska S., Bogdal D., Zaccheria F., Ravasio N., “The role of catalysis in the synthesis of polyurethane foams based on renewable raw materials”, Catalysis Today, 223 (2014) 148-156. DOI: 10.1016/j.cattod.2013.11.054.
[8] US patent number: 5039713, “Blowing reaction catalyst composition that provides cell opening of the resulting polyurethane foam”.
[9] The polyurethanes book, David Randall and Steve Lee, John Wiley & Sons, Ltd.
[10] Fent K., “Ecotoxicology of organotin compounds”, Critical Reviews in Toxicology, 26(1) (1996) 1-117.
[11] Hoch M., “Organotin compounds in the environment- an overview”, Applied Geochemistry, 16 (2001) 719-743. DOI: 10.1016/S0883-2927(00)00067-6.
[12] Sunday A., Alafara B., Oladele O., “Toxicity and speciation analysis of organotin compounds”, Chemical Speciation & Bioavailability, 24 (2012) 216-226. DOI: 10.3184/095422912X13491962881734.
[13] Official Journal of the European Union, Regulation (EC) No 1907/2006 of the European Parliament and of the Council (18 Dec 2006); REACH regulation.
[14] Nakashima H., Tomiyama K., Kawakami T., Isama K., “Analytical method for tributyltin and triphenyltin contained in household products- preparing for the revision of authorized analytical method”, The Pharmaceutical Society of Japan, 130(7) (2010) 945-954. DOI: 10.1248/yakushi.130.945.
[15] https://www.chromatographyonline.com/view/investigating-the-environmental-impact-of-organotins. Accessed on 01 February 2025.
[16] https://www.nihs.go.jp/mhlw/chemical/katei/PDF/orders_harmful_household_en_hold.pdf. Accessed on 01 February 2025.
[17] Korean Special Act on the Safety Control of Children’s Products, https://www.europeanqualitystandard.com/eqs/wp-content/uploads/2016/12/Corea_Nuovo-regolamento-prodotti-infanzia.pdf. Accessed on 01 February 2025.
[18] Legislation Workplace exposure standards for airborne contaminants (2024) https://www.safeworkaustralia.gov.au/sites/default/files/2024-01/workplace_exposure_standards_for_airborne_contaminants_-_18_january_2024.pdf. Accessed on 01 February 2025.
